Current issue
Online first
About
About the Journal
Aims & Scope
Editorial office
Abstracting and indexing
Publisher
Contact
For Authors
Author guideline
Manuscript template
Title page
Policies
Peer review policy
Ethical principles and publication policy
Language policy
Conflict of interest statement
Copyright statement
Open access policy
Archiving policy
Privacy statement
Referee and editor's guide
Price policy
License
Archive
BIOTECHNOLOGY & APPLIED MICROBIOLOGY / REVIEW
Advances in boron compounds: Author's perspectives on their role in biotechnology from antimicrobial agents to cancer therapy
1
Balikesir University, Chemistry, Savaştepe Vocational School, Turkey
Submission date: 2025-02-06
Final revision date: 2025-03-20
Acceptance date: 2025-03-20
Online publication date: 2025-04-15
Publication date: 2025-07-10
Euchembioj Rev. 1(2), Article e25009 (2025)
HIGHLIGHTS
- Boron compounds exhibit significant potential in medicinal chemistry.
- Structural modifications and functionalization are expanding their applications.
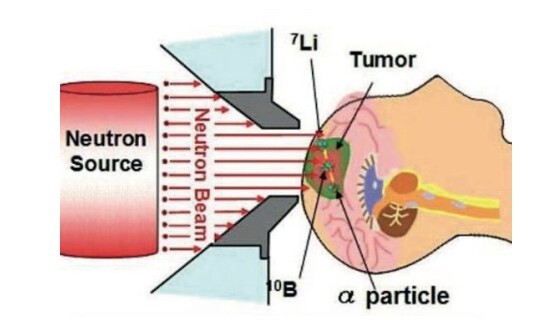
- BNCT is a targeted cancer treatment strategy with developing drug delivery systems.
KEYWORDS
enzyme inhibitionantimicrobial effectsboron compoundsbiotechnological applicationsboron neutron capture therapy
TOPICS
ABSTRACT
Boron compounds, both organic and inorganic, have emerged as versatile and promising materials with wide-ranging applications in medicinal chemistry, catalysis, and materials science. Organic boron compounds, including heterocyclic aminoboron derivatives and boronic acids, have shown significant potential as antimicrobial and anticancer agents, with research highlighting their effectiveness in treating infections and inhibiting cancer cell proliferation. The ongoing research, including the author's own studies, demonstrates significant promise regarding inorganic boron compounds, which possess a considerable potential that cannot be overlooked. Boron Neutron Capture Therapy (BNCT) has garnered attention for its targeted approach in cancer therapy, facilitated by the development of innovative boron-based drug delivery systems. Inorganic boron compounds, on the other hand, have contributed to advancements in catalytic processes, material stability, and electronic properties, offering opportunities for applications in organic electronics, flame-retardant materials, and drug development. The unique chemical reactivity of boron compounds, including their ability to inhibit enzymes such as β-lactamases and histone deacetylases, positions them as valuable tools in combating antibiotic resistance and cancer. This review provides a comprehensive overview of the properties, applications, and therapeutic potential of boron compounds, emphasizing their role in drug delivery, enzyme inhibition, and antimicrobial development. Ongoing research into the structural modification and functionalization of boron-based compounds continues to expand their scope, positioning them as key candidates for the development of novel therapeutic agents in biotechnology and medicine.
ACKNOWLEDGEMENTS
None.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest.
PEER REVIEW INFORMATION
Article has been screened for originality
Externally peer reviewed.
REFERENCES (55)
1.
Adamczyk-Woźniak, A., Borys, K. M., & Sporzyński, A. (2016). Recent developments in the chemistry and biological applications of boronic acids. Chemical Reviews, 116(9), 5083-5131.
2.
Adams, J. (2004). The proteasome: a suitable antineoplastic target. Nature Reviews Cancer, 4(5), 349-360. https://doi.org/10.1038/nrc136....
3.
Ahrens, V., Frank, R., Boehnke, S., Schütz, C., Hampel, G., Iffland, D., Bings, N.H., Hey-Hawkins, E.H., & Beck-Sickinger, A. (2014). Receptor-mediated uptake of boron-rich neuropeptide y analogues for boron neutron capture therapy. Chemmedchem, 10(1), 164-172. https://doi.org/10.1002/cmdc.2....
4.
Akbari, N., Ostadrahimi, A., Tutunchi, H., Pourmoradian, S., Farrin, N., Najafipour, F., Soleimanzadeh, H., Kafil, B., & Mobasseri, M. (2022). Possible therapeutic effects of boron citrate and oleoylethanolamide supplementation in patients with covid-19: a pilot randomized, doubleblind, clinical trial. Journal of Trace Elements in Medicine and Biology, 71, 126945. https://doi.org/10.1016/j.jtem....
5.
Ali, F., Hosmane, N., & Zhu, Y. (2020). Boron chemistry for medical applications. Molecules, 25(4), 828. https://doi.org/10.3390/molecu....
6.
Ando, N., Yamada, T., Narita, H., Oehlmann, N., Wagner, M., & Yamaguchi, S. (2021). Boron-doped polycyclic π-electron systems with an antiaromatic borole substructure that forms photoresponsive B–P Lewis adducts. Journal of the American Chemical Society, 143(26), 9944-9951. https://doi.org/10.1021/jacs.1....
7.
Anufriev, S., Sivaev, I., & Nakamura, H. (2020). Two possible ways to combine boron and gadolinium for Gd-guided BNCT: A concept. Phosphorus Sulfur and Silicon and the Related Elements, 195(11), 910-917. https://doi.org/10.1080/104265....
8.
Ailuno, G., Balboni, A., Caviglioli, G., Lai, F., Barbieri, F., Dellacasagrande, I., Florio, T., & Baldassari, S. (2022). Boron vehiculating nanosystems for neutron capture therapy in cancer treatment. Cells, 11(24), 4029. https://doi.org/10.3390/cells1....
9.
Barth, R., Mi, P., & Yang, W. (2018a). Boron delivery agents for neutron capture therapy of cancer. Cancer Communications, 38(1), 1-15. https://doi.org/10.1186/s40880....
10.
Barth, R., Zhang, Z., & Liu, T. (2018b). A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Communications, 38(1), 1-7. https://doi.org/10.1186/s40880....
11.
Brem, J., Cain, R., Cahill, S., McDonough, M., Clifton, I., Jiménez-Castellanos, J., Avison, M.B., Spencer, J., Fishwick, C.W.G., & Schofield, C. (2016). Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nature Communications, 7(1). https://doi.org/10.1038/ncomms....
12.
Cahill, S., Cain, R., Wang, D., Lohans, C., Wareham, D., Oswin, H., Mohammed, J., Spencer, J., Fishwick, C.W.G., McDonough, M.A., Schofield, C.J., & Brem, J. (2017). Cyclic boronates inhibit all classes of β-lactamases. Antimicrobial Agents and Chemotherapy, 61(4). https://doi.org/10.1128/aac.02....
13.
Campbell-Verduyn, L., Bowes, E., Li, H., Vallée, A., Vogels, C., Decken, A., Gray, C.A., & Westcott, S. (2014). Heterocyclic aminoboron compounds as antituberculosis agents. Heteroatom Chemistry, 25(2), 100-106. https://doi.org/10.1002/hc.211....
14.
Das, B. C., Nandwana, N. K., Das, S., Nandwana, V., Shareef, M. A., Das, Y., Saito, M., Weiss, L. M., Almaguel, F., Hosmane, N. S., & Evans, T. (2022). Boron chemicals in drug discovery and development: synthesis and medicinal perspective. Molecules, 27(9), 2615. https://doi.org/10.3390/molecu....
15.
Dash, B., Satapathy, R., Bode, B., Reidl, C., Sawicki, J., Mason, A., Maguire, J.A., & Hosmane, N. (2012). “Click” chemistry-mediated phenylene-cored carborane dendrimers. Organometallics, 31(7), 2931-2935. https://doi.org/10.1021/om2012....
16.
Fontaine, F., Héquet, A., Voisin-Chiret, A., Bouillon, A., Lesnard, A., Cresteil, T., Jolivalt, C., & Rault, S. (2014). First identification of boronic species as novel potential inhibitors of the Staphylococcus aureus Nora efflux pump. Journal of Medicinal Chemistry, 57(6), 2536-2548. https://doi.org/10.1021/jm4018....
17.
Frase, H., & Lee, I. (2007). Peptidyl boronates inhibit Salmonella enterica serovar Typhimurium Lon protease by a competitive ATP-dependent mechanism. Biochemistry, 46(22), 6647-6657. https://doi.org/10.1021/bi7002....
18.
Ganbar, G. (2019). Functionally substituted chemical organic compounds: Potential antimicrobial substances. Open Access Journal of Microbiology & Biotechnology, 4(1). https://doi.org/10.23880/oajmb....
19.
Glynn, S., Gaffney, K., Sainz, M., Louie, S., & Petasis, N. (2015). Molecular characterization of the boron adducts of the proteasome inhibitor bortezomib with epigallocatechin-3-gallate and related polyphenols. Organic & Biomolecular Chemistry, 13(13), 3887-3899. https://doi.org/10.1039/c4ob02....
20.
Hall, D.G. (2011). Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, Second Edition. Edited by Wiley-VCH Verlag GmbH & Co. KGaA. Hattori, Y., Ishimura, M., Ohta, Y., Takenaka, H., Kawabata, S., & Kirihata, M. (2021). Dodecaborate conjugates targeting tumor cell overexpressing translocator protein for boron neutron capture therapy. Acs Medicinal Chemistry Letters, 13(1), 50-54. https://doi.org/10.1021/acsmed....
21.
Heber, E., Hawthorne, M., Kueffer, P., Garabalino, M., Thorp, S., Pozzi, E., Hughes, A.M., Maitz, C.A., Jalisatgi, S.S., Nigg, D.W., Curotto, P., Trivillin, V.A., & Schwint, A. (2014). Therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes for oral cancer in the hamster cheek pouch model. Proceedings of the National Academy of Sciences, 111(45), 16077-16081. https://doi.org/10.1073/pnas.1....
22.
Heber, E., Kueffer, P., Lee, M., Hawthorne, M., Garabalino, M., Molinari, A., Nigg, D.W., Bauer, W., Hughes, A.M., Pozzi, E.C.C., Trivillin, V.A., & Schwint, A. (2012). Boron delivery with liposomes for boron neutron capture therapy (BNCT): Biodistribution studies in an experimental model of oral cancer demonstrating therapeutic potential. Radiation and Environmental Biophysics, 51(2), 195-204. https://doi.org/10.1007/s00411....
23.
Heide, F., McDougall, M., Harder-Viddal, C., Roshko, R., Davidson, D., Wu, J., Aprosoff, C., MoyaTorres, A., Lin, F.,& Stetefeld, J. (2021). Boron rich nanotube drug carrier system is suited for boron neutron capture therapy. Scientific Reports, 11(1). https://doi.org/10.1038/s41598....
24.
Issa, F., Kassiou, M., & Rendina, L. (2011). Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chemical Reviews, 111(9), 5701-5722. https://doi.org/10.1021/cr2000....
25.
Kaniowski, D., Suwara, J., Ebenryter-Olbińska, K., Jakóbik-Kolon, A., & Nawrot, B. (2022). EGFRtargeted cellular delivery of therapeutic nucleic acids mediated by boron clusters. International Journal of Molecular Sciences, 23(23), 14793. https://doi.org/10.3390/ijms23....
26.
Koganei, H., Ueno, M., Tachikawa, S., Tasaki, L., Ban, H., Suzuki, M., Shiraishi, K., Kawano, K., Yokoyama, M., Maitani, Y., Ono, K., & Nakamura, H. (2012). Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjugate Chemistry, 24(1), 124-132. https://doi.org/10.1021/bc3005....
27.
Koldemir-Gündüz, M., Aydın, H., Berikten, D., Kaymak, G., Köse, D., & Arslantaş, A. (2021). Synthesis of new boron derived compounds; anticancer, antioxidant and antimicrobial effect in vitro glioblastoma tumor model. Journal of Korean Neurosurgical Society, 64(6), 864-872. https://doi.org/10.3340/jkns.2....
28.
Kollár, L. (2024). Boronic acid inhibitors of penicillin-binding protein 1b: Serine and lysine labelling agents. Journal of Enzyme Inhibition and Medicinal Chemistry, 39(1). https://doi.org/10.1080/147563....
29.
Krajnc, A., Brem, J., Hinchliffe, P., Calvopiña, K., Panduwawala, T., Lang, P., Kamps, J.J.A.G., Tyrrell, J.M., Widlake, E., Saward, B.G., Walsh, T.R., Spencer, J., & Schofield, C. (2019). Bicyclic boronate VNRX-5133 inhibits metallo- and serine-β-lactamases. Journal of Medicinal Chemistry, 62(18), 8544-8556. https://doi.org/10.1021/acs.jm....
30.
Li, X., Wang, X., Zhang, J., Hanagata, N., Wang, X., Weng, Q., Ito, A., Bando, Y., & Golberg, D. (2017). Hollow boron nitride nanospheres as boron reservoir for prostate cancer treatment. Nature Communications, 8(1). https://doi.org/10.1038/ncomms....
31.
Luderer, M., Muz, B., Puente, P., Chavalmane, S., Kapoor, V., Marcelo, R., Biswas, P., Thotala, D., Rogers, B., & Azab, A. (2016). A hypoxia-targeted boron neutron capture therapy agent for the treatment of glioma. Pharmaceutical Research, 33(10), 2530-2539. https://doi.org/10.1007/s11095....
32.
Milani, P., Demasi, M., Rezende, L., Amaral, A., & Andrade, L. (2014). Synthesis of L-cysteine-based boron compounds and their evaluation as proteasome inhibitors. New Journal of Chemistry, 38(10), 4859-4871. https://doi.org/10.1039/c4nj00....
33.
Mergheş, P., Ilia, G., Hulka, I., Chiriac, V., Varan, N., & Simulescu, V. (2022). The influence of boron on the structure and properties of hybrid compounds containing zirconium and phosphorus. Gels, 8(10), 667. https://doi.org/10.3390/gels81....
34.
El-Zaria, M., & Nakamura, H. (2009). New strategy for synthesis of mercaptoundecahydrododecaborate derivatives via click chemistry: possible boron carriers and visualization in cells for neutron capture therapy. Inorganic Chemistry, 48(24), 11896-11902. https://doi.org/10.1021/ic9020....
35.
Nakase, I., Aoki, A., Sakai, Y., Hirase, S., Ishimura, M., Takatani-Nakase, T., Hattori, Y., & Kirihata, M. (2020). Antibody-based receptor targeting using an FC-binding peptide-dodecaborate conjugate and macropinocytosis induction for boron neutron capture therapy. Acs Omega, 5(36), 22731- 22738. https://doi.org/10.1021/acsome....
36.
Saraç, N., Uğur, A., Boran, R., & Elgin, E. (2015). The use of boron compounds for stabilization of lipase from Pseudomonas aeruginosa ES3 for the detergent industry. Journal of Surfactants and Detergents, 18(2), 275-285. https://doi.org/10.1007/s11743....
37.
Scorei, I. and Popa, R. (2010). Boron-containing compounds as preventive and chemotherapeutic agents for cancer. Anti-Cancer Agents in Medicinal Chemistry, 10(4), 346-351. https://doi.org/10.2174/187152....
38.
Seneviratne, D., Saifi, O., Mackeyev, Y., Malouff, T., & Krishnan, S. (2023). Next-generation boron drugs and rational translational studies driving the revival of bnct. Cells, 12(10), 1398. https://doi.org/10.3390/cells1....
39.
Suzuki, N., Suzuki, T., Ota, Y., Nakano, T., Kurihara, M., Okuda, H., Yamori, T., Tsumoto, H., Nakagawa, H., & Miyata, N. (2009). Design, synthesis, and biological activity of boronic acidbased histone deacetylase inhibitors. Journal of Medicinal Chemistry, 52(9), 2909-2922. https://doi.org/10.1021/jm9001....
40.
Tamanoi, F., Chinnathambi, S., Laird, M., Komatsu, A., Birault, A., Takata, T., Doan, T. L.-H., Mai, N. X. D., Raitano, A., Morrison, K., Suzuki, M., & Matsumoto, K. (2021). Construction of boronophenylalanine-loaded biodegradable periodic mesoporous organosilica nanoparticles for bnct cancer therapy. International Journal of Molecular Sciences, 22(5), 2251. https://doi.org/10.3390/ijms22....
41.
Thamilselvan, G., Sarveswari, H., Vasudevan, S., Stanley, A., Shanmugam, K., Vairaprakash, P., & Solomon, A.P. (2021). Development of an antibiotic resistance breaker to resensitize drugresistant staphylococcus aureus: in silico and in vitro approach. Frontiers in Cellular and Infection Microbiology, 11. https://doi.org/10.3389/fcimb.....
42.
Türkez, H., Geyikoğlu, F., Tatar, A., Keleş, M., & Özkan, A. (2007). Effects of some boron compounds on peripheral human blood. Zeitschrift Für Naturforschung C, 62(11-12), 889-896. https://doi.org/10.1515/znc-20....
43.
Unlu, S., Doğan, Ş., & Doğan, M. (2014). Comparative study of boron compounds and aluminum trihydroxide as flame retardant additives in epoxy resin. Polymers for Advanced Technologies, 25(8), 769-776. https://doi.org/10.1002/pat.32....
44.
Vedelago, J., Mattea, F., Triviño, S., Montesinos, M., Keil, W., Valente, M., & Romero, M. (2021). Smart material based on boron crosslinked polymers with potential applications in cancer radiation therapy. Scientific Reports, 11(1). https://doi.org/10.1038/s41598....
45.
Wang, S., Blaha, C., Santos, R., Huynh, T., Hayes, T., Beckford-Vera, D., Blecha, J.E., Hong, A.S., Miko Fogarty, Hope, T.A., Raleigh, D.R., Wilson, D.M., Evans, M.J., VanBrocklin, H.F., Ozawa, T., & Flavell, R. (2019). Synthesis and initial biological evaluation of boron-containing prostate-specific membrane antigen ligands for treatment of prostate cancer using boron neutron.
47.
Wang, Y. and Spokoyny, A. (2022). Abiotic main group pharmacophore renders a new class of antimicrobial agents. Acs Central Science, 8(3), 309-311. https://doi.org/10.1021/acscen....
48.
Worm, D., Els-Heindl, S., Kellert, M., Kuhnert, R., Saretz, S., Koebberling, J., Riedl, B., Hey-Hawkins, E., & Beck-Sickinger, A. (2018). A stable meta-carborane enables the generation of boron-rich peptide agonists targeting the ghrelin receptor. Journal of Peptide Science, 24(10). https://doi.org/10.1002/psc.31....
49.
Worm, D., Hoppenz, P., Els-Heindl, S., Kellert, M., Kuhnert, R., Saretz, S., Köbberling, J., Riedl, B., Hey-Hawkins, E., & Beck-Sickinger, A. (2019). Selective neuropeptide y conjugates with maximized carborane loading as promising boron delivery agents for boron neutron capture therapy. Journal of Medicinal Chemistry, 63(5), 2358-2371. https://doi.org/10.1021/acs.jm....
50.
Xiong, H., Wang, X., Zhou, D., Qi, Y., Xie, Z., Chen, X., Jing, X., & Huang, Y. (2016). Amphiphilic polycarbonates from carborane-installed cyclic carbonates as potential agents for boron neutron capture therapy. Bioconjugate Chemistry, 27(9), 2214-2223. https://doi.org/10.1021/acs.bi....
51.
Yang, W., Gao, X., & Wang, B. (2003). Boronic acid compounds as potential pharmaceutical agents. Medicinal Research Reviews, 23(3), 346-368. https://doi.org/10.1002/med.10....
52.
Yuan, Z., Zhang, J., Guo, J., Xu, Y., Duan, K., Zheng, J., Wan, H., Yuan, Z., & Chen, H. (2019). Application of nitroimidazole–carbobane-modified phenylalanine derivatives as dual-target boron carriers in boron neutron capture therapy. Molecular Pharmaceutics, 17(1), 202-211. https://doi.org/10.1021/acs.mo....
53.
Zhi, S., Wang, Y., Li, Y., Cheng, J., & Yang, G. (2018). Linking 1d transition-metal coordination polymers and different inorganic boron oxides to construct a series of 3d inorganic–organic hybrid borates. Inorganic Chemistry, 57(3), 1350-1355. https://doi.org/10.1021/acs.in....
54.
Zhu, D., Hunter, C., Baird, S., Davis, B., Bos, A., Geier, S., Vogels, C.M., Decken, A., Gray, C.A., & Westcott, S. (2017). Synthesis and antimicrobial properties of cyclic fluorodiamines containing boronate esters. Heteroatom Chemistry, 28(6). https://doi.org/10.1002/hc.214....
55.
Zu, B., Guo, Y., & He, C. (2021). Catalytic enantioselective construction of chiroptical boronstereogenic compounds. Journal of the American Chemical Society, 143(39), 16302-16310.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.