Unraveling Epilepsy: Investigating stem cell approaches for innovative treatment and future cure
DOI:
https://doi.org/10.62063/rev-11Keywords:
Epilepsy, Treatment, Neurological disorders, Neural networks, Seizures, Stem cellAbstract
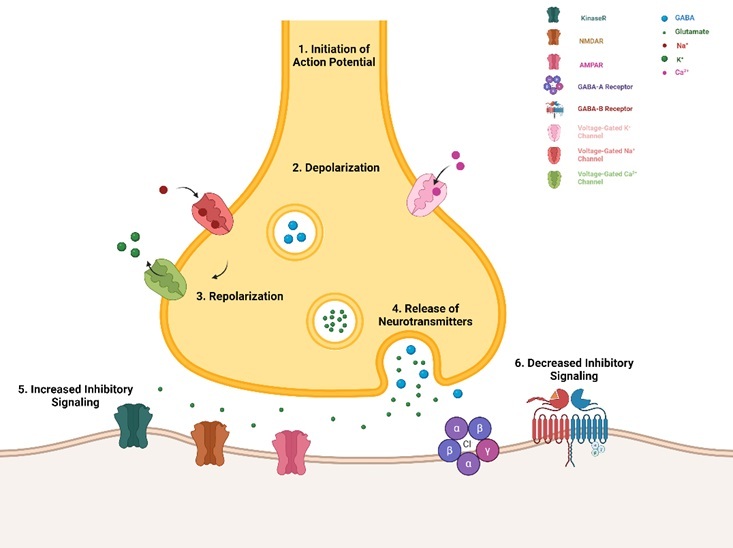
Epilepsy is a persistent neurological disorder characterized by repeated, spontaneous seizures that arise without a specific cause. These seizures result from abnormal electrical activity in the brain, leading to a range of symptoms, from brief periods of unconsciousness or minor sensory disturbances to severe convulsions. The management of epilepsy remains a significant challenge, as current treatment modalities, primarily involving antiepileptic drugs and surgical interventions to remove seizure foci, often provide adequate control for a substantial portion of patients. For this reason, stem cell therapies have become a hopeful approach because of their ability to potentially restore and renew impaired neural networks, which is particularly relevant for neurological disorders like epilepsy. This review investigates the present state of stem cell therapies in epilepsy, analyzing distinct types of stem cells, their mode of action, preclinical and clinical trials, as well as future research prospects.
References
Abdanipour, A., Tiraihi, T., & Mirnajafi-Zadeh, J. (2011). Improvement of the pilocarpine epilepsy model in rat using bone marrow stromal cell therapy. Neurological research, 33(6), 625–632. https://doi.org/10.1179/1743132810Y.0000000018
Alayli, A., Lockard, G., Gordon, J., Connolly, J., Monsour, M., Schimmel, S., Dela Peña, I., & Borlongan, C. V. (2023). Stem Cells: Recent Developments Redefining Epilepsy Therapy. Cell transplantation, 32, 9636897231158967. https://doi.org/10.1177/09636897231158967
Bond, A. M., Ming, G. L., & Song, H. (2015). Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell stem cell, 17(4), 385–395. https://doi.org/10.1016/j.stem.2015.09.003
Chang, B. L., & Chang, K. H. (2022). Stem Cell Therapy in Treating Epilepsy. Frontiers in neuroscience, 16, 934507. https://doi.org/10.3389/fnins.2022.934507
DaCosta, J. C., Portuguez, M. W., Marinowic, D. R., Schilling, L. P., Torres, C. M., DaCosta, D. I., Carrion, M. J. M., Raupp, E. F., Machado, D. C., Soder, R. B., Lardi, S. L., & Garicochea, B. (2018). Safety and seizure control in patients with mesial temporal lobe epilepsy treated with regional superselective intra-arterial injection of autologous bone marrow mononuclear cells. Journal of tissue engineering and regenerative medicine, 12(2), e648–e656. https://doi.org/10.1002/term.2334
Das, M., Mayilsamy, K., Mohapatra, S. S., & Mohapatra, S. (2019). Mesenchymal stem cell therapy for the treatment of traumatic brain injury: progress and prospects. Reviews in the neurosciences, 30(8), 839–855. https://doi.org/10.1515/revneuro-2019-0002
Datta, D., Subburaju, S., Kaye, S., Baruah, J., Choi, Y. K., Nian, Y., Khalili, J. S., Chung, S., Elkhal, A., & Vasudevan, A. (2020). Human forebrain endothelial cell therapy for psychiatric disorders. Molecular Psychiatry 2020 26:9, 26(9), 4864–4883. https://doi.org/10.1038/s41380-020-0839-9
de Gois da Silva, M. L., da Silva Oliveira, G. L., de Oliveira Bezerra, D., da Rocha Neto, H. J., Feitosa, M. L. T., Argôlo Neto, N. M., Rizzo, M. D. S., & de Carvalho, M. A. M. (2018). Neurochemical properties of neurospheres infusion in experimental-induced seizures. Tissue & cell, 54, 47–54. https://doi.org/10.1016/j.tice.2018.08.002
Dubé, C. M., Brewster, A. L., Richichi, C., Zha, Q., & Baram, T. Z. (2007). Fever, febrile seizures and epilepsy. Trends in neurosciences, 30(10), 490–496. https://doi.org/10.1016/J.TINS.2007.07.006
French, J. A., Kanner, A. M., Bautista, J., Abou-Khalil, B., Browne, T., Harden, C. L., Theodore, W. H., Bazil, C., Stern, J., Schachter, S. C., Bergen, D., Hirtz, D., Montouris, G. D., Nespeca, M., Gidal, B., Marks, W. J., Turk, W. R., Fischer, J. H., Bourgeois, B., … Glauser, T. A. (2004). Efficacy and Tolerability of the New Antiepileptic Drugs, I: Treatment of New-Onset Epilepsy: Report of the TTA and QSS Subcommittees of the American Academy of Neurology and the American Epilepsy Society. Epilepsia, 45(5), 401–409. https://doi.org/10.1111/J.0013-9580.2004.06204.X
Fukumura, S., Sasaki, M., Kataoka-Sasaki, Y., Oka, S., Nakazaki, M., Nagahama, H., Morita, T., Sakai, T., Tsutsumi, H., Kocsis, J. D., & Honmou, O. (2018). Intravenous infusion of mesenchymal stem cells reduces epileptogenesis in a rat model of status epilepticus. Epilepsy Research, 141, 56–63. https://doi.org/10.1016/J.EPLEPSYRES.2018.02.008
Guerreiro, C. A. M. (2016). Epilepsy: Is there hope? The Indian Journal of Medical Research, 144(5), 657. https://doi.org/10.4103/IJMR.IJMR_1051_16
Hirose, S., Tanaka, Y., Shibata, M., Kimura, Y., Ishikawa, M., Higurashi, N., Yamamoto, T., Ichise, E., Chiyonobu, T., & Ishii, A. (2020). Application of induced pluripotent stem cells in epilepsy. Molecular and Cellular Neuroscience, 108, 103535. https://doi.org/10.1016/J.MCN.2020.103535
Hlebokazov, F., Dakukina, T., Ihnatsenko, S., Kosmacheva, S., Potapnev, M., Shakhbazau, A., Goncharova, N., Makhrov, M., Korolevich, P., Misyuk, N., Dakukina, V., Shamruk, I., Slobina, E., & Marchuk, S. (2017). Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: An open label study. Advances in Medical Sciences, 62(2), 273–279. https://doi.org/10.1016/J.ADVMS.2016.12.004
Hlebokazov, F., Dakukina, T., Potapnev, M., Kosmacheva, S., Moroz, L., Misiuk, N., Golubeva, T., Slobina, E., Krasko, O., Shakhbazau, A., Hlavinski, I., & Goncharova, N. (2021). Clinical benefits of single vs repeated courses of mesenchymal stem cell therapy in epilepsy patients. Clinical Neurology and Neurosurgery, 207. https://doi.org/10.1016/J.CLINEURO.2021.106736
Huang, P. Y., Shih, Y. H., Tseng, Y. J., Ko, T. L., Fu, Y. S., & Lin, Y. Y. (2016). Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain, behavior, and immunity, 54, 45–58. https://doi.org/10.1016/j.bbi.2015.12.021
Jiang, M., Ye, J., Wang, X., Li, N., Wang, Y., & Shi, Y. (2020). Phosphatase SHP1 impedes mesenchymal stromal cell immunosuppressive capacity modulated by JAK1/STAT3 and P38 signals. Cell and Bioscience, 10(1), 1–10.
https://doi.org/10.1186/S13578-020-00428-W/FIGURES/4
Johnson, E. L. (2019). Seizures and Epilepsy. The Medical Clinics of North America, 103(2), 309-324. https://doi.org/10.1016/J.MCNA.2018.10.002
Klein, P., Kaminski, R. M., Koepp, M., & Löscher, W. (2024). New epilepsy therapies in development. Nature Reviews. Drug Discovery. https://doi.org/10.1038/S41573-024-00981-W
Kodali, M., Castro, O. W., Kim, D. K., Thomas, A., Shuai, B., Attaluri, S., Upadhya, R., Gitai, D., Madhu, L. N., Prockop, D. J., & Shetty, A. K. (2019). Intranasally Administered Human MSC Derived Extracellular Vesicles Pervasively Incorporate into Neurons and Microglia in both Intactand Status Epilepticus Injured Forebrain. International Journal of Molecular Sciences 2020, Vol. 21, Page 181, 21(1), 181. https://doi.org/10.3390/IJMS21010181
Kwan, P., Arzimanoglou, A., Berg, A. T., Brodie, M. J., Hauser, W. A., Mathern, G., Moshé, S. L., Perucca, E., Wiebe, S., & French, J. (2010). Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia, 51(6), 1069–1077. https://doi.org/10.1111/J.1528-1167.2009.02397.X
Kwan, P., Schachter, S. C., & Brodie, M. J. (2011). Drug-resistant epilepsy. New England Journal of Medicine, 365(10), 919–926. https://doi.org/https://doi.org/10.1056/nejmra1004418
Lee, V. L. L., Choo, B. K. M., Chung, Y. S., Kundap, U. P., Kumari, Y., & Shaikh, M. F. (2018). Treatment, Therapy and Management of Metabolic Epilepsy: A Systematic Review. International Journal of Molecular Sciences, 19(3). https://doi.org/10.3390/IJMS19030871
Liu, G., Health, A., Slater, N., & Perkins, A. (2017). Epilepsy: Treatment Options. American Family Physician, 96(2), 87–96. https://www.aafp.org/pubs/afp/issues/2017/0715/p87.html
Liu, Z. Z., Huang, Y., Hong, C. G., Wang, X., Duan, R., Liu, J. Y., He, J. L., Duan, D., Xie, H., & Lu, M. (2023). Autologous olfactory mucosa mesenchymal stem cells treatment improves the neural network in chronic refractory epilepsy. Stem Cell Research and Therapy, 14(1), 1–16. https://doi.org/10.1186/S13287-023-03458-6/FIGURES/8
St Louis E. K. (2009). Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Current neuropharmacology, 7(2), 96–105. https://doi.org/10.2174/157015909788848929
Lybrand, Z. R., Goswami, S., & Hsieh, J. (2020). Stem cells: a path towards improved epilepsy therapies. Neuropharmacology, 168, 107781. https://doi.org/10.1016/J.NEUROPHARM.2019.107781
McNamara, J. O. (1999). Emerging insights into the genesis of epilepsy. Nature, 399(6738 Suppl). https://doi.org/10.1038/399A015
Miguel Sanz, C., Martinez Navarro, M., Caballero Diaz, D., Sanchez-Elexpuru, G., & di Donato, V. (2023). Toward the use of novel alternative methods in epilepsy modeling and drug discovery. Frontiers in Neurology, 14, 1213969. https://doi.org/10.3389/FNEUR.2023.1213969/BIBTEX
Milczarek, O., Jarocha, D., Starowicz–Filip, A., Kwiatkowski, S., Badyra, B., & Majka, M. (2018). Multiple Autologous Bone Marrow–Derived CD271+ Mesenchymal Stem Cell Transplantation Overcomes Drug–Resistant Epilepsy in Children. Stem Cells Translational Medicine, 7(1), 20. https://doi.org/10.1002/SCTM.17-0041
Mohammed, A. S., Ewais, M. M., Tawfik, M. K., & Essawy, S. S. (2014). Effects of intravenous human umbilical cord blood mesenchymal stem cell therapy versus gabapentin in pentylenetetrazole-induced chronic epilepsy in rats. Pharmacology, 94(1–2), 41–50. https://doi.org/10.1159/000365219
Murray, C. J. L., Vos, T., Lozano, R., Naghavi, M., Flaxman, A. D., Michaud, C., Ezzati, M., Shibuya, K., Salomon, J. A., Abdalla, S., Aboyans, V., Abraham, J., Ackerman, I., Aggarwal, R., Ahn, S. Y., Ali, M. K., AlMazroa, M. A., Alvarado, M., Anderson, H. R., … Lopez, A. D. (2012). Disability adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England), 380(9859), 2197–2223. https://doi.org/10.1016/S0140-6736(12)61689-4
Nair, D. R. (2016). Management of Drug-Resistant Epilepsy. Continuum (Minneapolis, Minn.), 22(1Epilepsy), 157–172. https://doi.org/10.1212/CON.0000000000000297
Neal, E. G., Chaffe, H., Schwartz, R. H., Lawson, M. S., Edwards, N., Fitzsimmons, G., Whitney, A., & Cross, J. H. (2008). The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. The Lancet Neurology, 7(6), 500–506. https://doi.org/10.1016/S14744422(08)70092-9
Papazian, I., Kyrargyri, V., Evangelidou, M., Voulgari-Kokota, A., & Probert, L. (2018). Mesenchymal Stem Cell Protection of Neurons against Glutamate Excitotoxicity Involves Reduction of NMDA Triggered Calcium Responses and Surface GluR1, and Is Partly Mediated by TNF. International Journal of Molecular Sciences 2018, Vol. 19, Page 651, 19(3), 651. https://doi.org/10.3390/IJMS19030651
Patel, D. C., Tewari, B. P., Chaunsali, L., & Sontheimer, H. (2019). Neuron-glia interactions in the pathophysiology of epilepsy. Nature Reviews. Neuroscience, 20(5), 282–297. https://doi.org/10.1038/S41583-019-0126-4
Poppe, D., Doerr, J., Schneider, M., Wilkens, R., Steinbeck, J. A., Ladewig, J., Tam, A., Paschon, D. E., Gregory, P. D., Reik, A., Müller, C. E., Koch, P., & Brüstle, O. (2018). Genome Editing in Neuroepithelial Stem Cells to Generate Human Neurons with High Adenosine-Releasing Capacity. Stem Cells Translational Medicine, 7(6), 477–486. https://doi.org/10.1002/SCTM.16-0272
Riva, A., Golda, A., Balagura, G., Amadori, E., Vari, M. S., Piccolo, G., Iacomino, M., Lattanzi, S., Salpietro, V., Minetti, C., & Striano, P. (2021). New Trends and Most Promising Therapeutic Strategies for Epilepsy Treatment. Frontiers in Neurology, 12, 753753. https://doi.org/10.3389/FNEUR.2021.753753
Salem, N. A., El-Shamarka, M., Khadrawy, Y., & El-Shebiney, S. (2018). New prospects of mesenchymal stem cells for ameliorating temporal lobe epilepsy. Inflammopharmacology, 26(4), 963-972. https://doi.org/10.1007/S10787-018-0456-2
Sayed, N., Liu, C., & Wu, J. C. (2016). Translation of Human-Induced Pluripotent Stem Cells: From Clinical Trial in a Dish to Precision Medicine. Journal of the American College of Cardiology, 67(18), 2161–2176. https://doi.org/10.1016/J.JACC.2016.01.083
Shaefi, S., & Harkness, W. (2003). Current Status of Surgery in the Management of Epilepsy. Epilepsia, 44(SUPPL. 1), 43–47. https://doi.org/10.1046/J.1528-1157.44.S.1.15.X
Sirven J. I. (2015). Epilepsy: A Spectrum Disorder. Cold Spring Harbor perspectives in medicine, 5(9), a022848. https://doi.org/10.1101/cshperspect.a022848
Szczepanik, E., Mierzewska, H., Antczak-Marach, D., Figiel-Dabrowska, A., Terczynska, I., Tryfon, J., Krzesniak, N., Noszczyk, B. H., Sawicka, E., Domanska-Janik, K., & Sarnowska, A. (2020). Intrathecal Infusion of Autologous Adipose-Derived Regenerative Cells in Autoimmune Refractory Epilepsy: Evaluation of Safety and Efficacy. Stem Cells International, 2020. https://doi.org/10.1155/2020/7104243
Thodeson, D. M., Brulet, R., & Hsieh, J. (2018). Neural stem cells and epilepsy: functional roles and disease-in-a-dish models. Cell and Tissue Research, 371(1), 47–54. https://doi.org/10.1007S00441-017-2675-Z
Thompson, K., Kanemori, M., Kimes, E., Hobbs, E., Phan, J., Wilson, T., Wertheimer, A., Yoshida, T., Flores-Barnett, D., Kang, M., & Auyang, C. (2023). Transplantation of neurogenic-fusionogenic embryonic stem cells modified to overexpress GABA into a model of temporal lobe epilepsy: Promises and potential pitfalls. Medical Research Archives, 11(10). https://doi.org/10.18103/MRA.V11I10.4510
Upadhya, D., Hattiangady, B., Castro, O. W., Shuai, B., Kodali, M., Attaluri, S., Bates, A., Dong, Y., Zhang, S. C., Prockop, D. J., & Shetty, A. K. (2019). Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proceedings of the National Academy of Sciences of the United States of America, 116(1), 287–296. https://doi.org/10.1073/PNAS.1814185115/-/DCSUPPLEMENTAL
Vezzani, A., Balosso, S., & Ravizza, T. (2019). Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nature Reviews. Neurology, 15(8), 459–472. https://doi.org/10.1038/ S41582-019-0217-X
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J., & Perez-Fernandez, R. (2017). Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. International Journal of Molecular Sciences, 18(9). https://doi.org/10.3390/IJMS18091852
Voskuyl, R. A., & Clinckers, R. (2009). ANTIEPILEPTIC DRUGS | Pharmacological Approaches for the Assessment of Antiepileptic Drug Efficacy in Experimental Animal Models. Encyclopedia of Basic Epilepsy Research, 90–97. https://doi.org/10.1016/B978-012373961-2.00235-6
Wang, L., Zhao, Y., Pan, X., Zhang, Y., Lin, L., Wu, Y., Huang, Y., & He, H. (2021). Adipose-derived stem cell transplantation improves learning and memory via releasing neurotrophins in rat model of temporal lobe epilepsy. Brain Research, 1750. https://doi.org/10.1016/J.BRAINRES.2020.147121
Wesselschmidt, R. L. (2012). Embryonic Stem Cells and Neurogenesis. Neural Development and Stem Cells: Third Edition, 31–59. https://doi.org/10.1007/978-1-4614-3801-4_2
Xian, P., Hei, Y., Wang, R., Wang, T., Yang, J., Li, J., Di, Z., Liu, Z., Baskys, A., Liu, W., Wu, S., & Long, Q. (2019). Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics, 9(20), 5956–5975. https://doi.org/10.7150/THNO.33872
Xu, K., Liu, F., Xu, W., Liu, J., Chen, S., & Wu, G. (2019). Transplanting GABAergic Neurons Differentiated from Neural Stem Cells into Hippocampus Inhibits Seizures and Epileptiform Discharges in Pilocarpine-Induced Temporal Lobe Epilepsy Model. World Neurosurgery, 128, e1–e11. https://doi.org/10.1016/J.WNEU.2019.01.245
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Hilal Balcilar, Sajeda Osman, Sevim Isik

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







